CAR T-Cell Therapy Market Overview
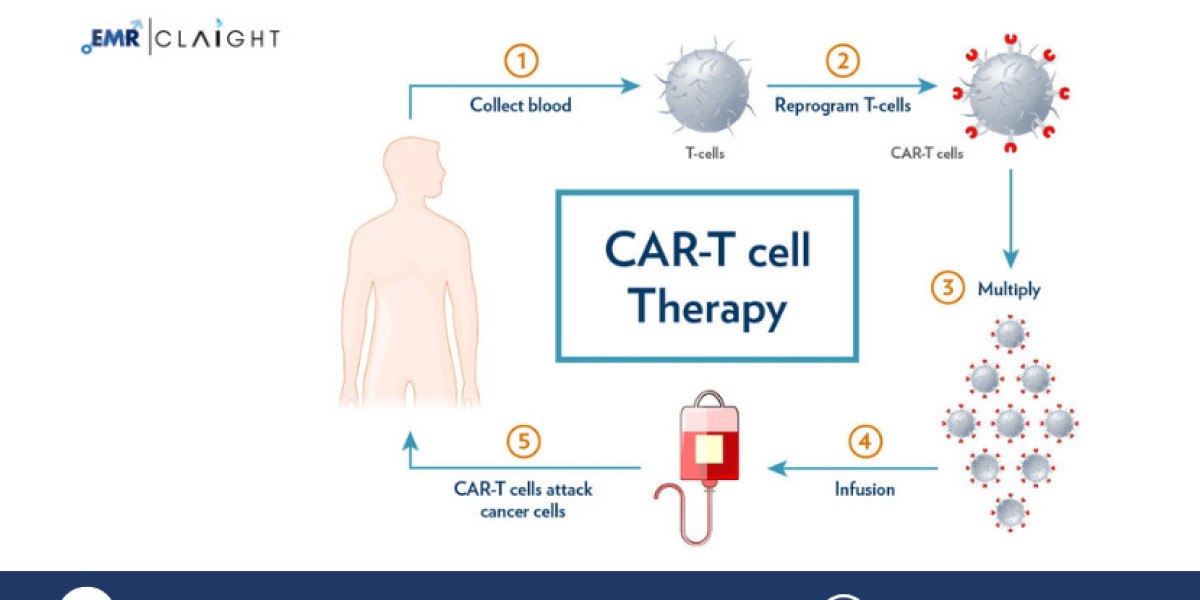
CAR T-cell therapy (Chimeric Antigen Receptor T-cell therapy) is an advanced form of immunotherapy that uses the body's immune cells to target and eliminate cancer cells. In this therapy, a patient's T-cells (a type of white blood cell) are modified in the laboratory to produce chimeric antigen receptors (CARs) on their surface. These receptors enable the T-cells to recognize and attack specific cancer cells. CAR T-cell therapy is particularly effective for hematologic (blood) cancers such as leukemia, lymphoma, and multiple myeloma, though its use is expanding to solid tumors.
The significance of CAR T-cell therapy lies in its potential to provide long-term remission for patients with cancers that were previously difficult to treat. CAR T-cell therapies are considered a breakthrough in oncology, offering hope for patients with limited treatment options, especially those who have relapsed or are resistant to conventional therapies. As of 2024, several CAR T-cell therapies have been approved by regulatory agencies such as the FDA, with ongoing clinical trials for new indications.
CAR T-Cell Therapy Market Size and Share
The CAR T-cell therapy market size was valued at USD 2.5 billion in 2024, marking significant growth from previous years. This growth is attributed to increasing cancer incidences and the ongoing advancements in CAR T-cell technologies. In 2025, the market is expected to grow to USD 2.9 billion, and it is projected to reach USD 9.0 billion by 2034, driven by an expected CAGR of 15.2%. The rapid adoption of CAR T-cell therapy, coupled with the high demand for effective cancer treatments, is expected to fuel this market's growth.
North America remains the largest market for CAR T-cell therapies, with the United States leading in terms of both revenue and the number of patients receiving treatment. Europe and Asia Pacific are also witnessing increased adoption due to the growing number of clinical trials and the expansion of healthcare infrastructures. In terms of market share, Gilead Sciences, Novartis, and other biotechnology companies play a dominant role in shaping the landscape of the CAR T-cell therapy market.
CAR T-Cell Therapy Market Trends
- Increasing Adoption of CAR T-Cell Therapies The adoption of CAR T-cell therapies has been increasing rapidly in the oncology field, especially for blood cancers. With the approval of therapies like Kymriah and Yescarta, more patients now have access to cutting-edge treatments that were previously not available. As the efficacy of these therapies becomes more evident, their use is likely to expand further, particularly in relapsed or refractory cancers.
- Expansion to Solid Tumors While CAR T-cell therapy has primarily been successful in treating hematologic cancers, ongoing research is focused on expanding its use to solid tumors. This area of research is vital as solid tumors, such as breast cancer, pancreatic cancer, and glioblastoma, represent a significant unmet medical need. Advances in CAR T-cell technology, such as better tumor penetration and overcoming the immunosuppressive environment of solid tumors, are expected to drive this trend.
- Personalization and Advanced Manufacturing Techniques One of the emerging trends in the CAR T-cell therapy market is the shift towards more personalized treatments. CAR T-cells are made from the patient’s own T-cells, making them a form of autologous therapy. However, advancements in allogeneic CAR T-cell therapies, where T-cells from a donor are used, are opening up new possibilities for more cost-effective and widely accessible treatments.
- Collaborations and Strategic Partnerships Pharmaceutical and biotechnology companies are increasingly forming partnerships to advance CAR T-cell therapies. Collaborations between large pharmaceutical companies and smaller biotech firms are accelerating the development and commercialization of new therapies. These partnerships also allow for the sharing of resources and expertise, expediting the progress of clinical trials and approval processes.
Access a Free Sample of the Insights CAR T-Cell Therapy Market
CAR T-Cell Therapy Market Analysis
- Scope of the Report The CAR T-cell therapy market report provides an in-depth analysis of the market's current and future performance. It covers the global market size, industry drivers, and trends, and includes detailed analysis of the key segments, including drug type, antigen, application, and region. The report also discusses the regulatory landscape, challenges, and opportunities in the CAR T-cell therapy space.
- Historical and Forecast Trends The historical trend shows a rapid evolution in CAR T-cell therapy, from the initial FDA approvals for blood cancers to the ongoing trials for solid tumors. Over the next decade, CAR T-cell therapies are expected to play an increasingly central role in oncology treatment. The market will experience robust growth, driven by continuous research, approval of new therapies, and increased patient access.
- Industry Drivers and Constraints The major drivers of the CAR T-cell therapy market include the rising incidence of cancer, technological advancements in CAR T-cell production, and a growing demand for personalized treatments. However, the market faces challenges such as high treatment costs, logistical issues in manufacturing and distribution, and potential side effects such as cytokine release syndrome (CRS) and neurotoxicity.
Historical and Forecast Market Analysis by Segment
- Drug Type: The CAR T-cell therapy market includes key drugs like Axicabtagene Ciloleucel, Tisagenlecleucel, Brexucabtagene Autoleucel, Lisocabtagene Maraleuce, Idecabtagene Vicleucel, and Ciltacabtegene Autoleucel. Each drug has its unique characteristics and applications, with some approved for specific cancers, while others are still in clinical trials.
- Antigen: The market is segmented by antigen targets, with key targets including CD19, BCMA, HER2, and GD2, among others. Each antigen plays a crucial role in targeting specific cancer cells.
- Application: CAR T-cell therapies are applied to treat various cancers, including Acute Lymphocytic Leukemia (ALL), Chronic Lymphocytic Leukemia (CLL), Diffuse Large B-Cell Lymphoma (DLBCL), and multiple myeloma. There is also ongoing research into using CAR T-cell therapy for solid tumors, such as breast and pancreatic cancer.
- Region: North America dominates the market, followed by Europe and Asia Pacific. The growth in emerging markets is fueled by increasing cancer prevalence and expanding healthcare infrastructure.
Regional Insights
- North America North America holds the largest market share in the CAR T-cell therapy sector. The U.S. is a leader in both the number of clinical trials and the approval of CAR T-cell therapies. The presence of major biotechnology firms such as Gilead Sciences, Novartis, and Kite Pharma further propels the growth of the market. The healthcare system in North America supports the adoption of advanced therapies like CAR T-cell treatments, offering significant growth potential in the region.
- Europe and Asia-Pacific Europe is experiencing significant growth due to the increasing approval of CAR T-cell therapies in the region and the growing number of cancer cases. Countries like Germany, France, and the UK are major contributors to the European CAR T-cell therapy market. Meanwhile, Asia-Pacific is emerging as a promising market for CAR T-cell therapies, particularly in countries like Japan, China, and India, where the incidence of cancer is rising and access to healthcare is improving.
Market Growth – Factors Driving Growth and Future Opportunities
The CAR T-cell therapy market is witnessing robust growth, fueled by a range of factors. The rising incidence of cancer, especially hematologic cancers, is a significant driver. Furthermore, increasing awareness among patients and healthcare providers about the benefits of CAR T-cell therapies is expected to accelerate market growth. Additionally, advancements in manufacturing processes, which make CAR T-cell therapies more accessible, will drive future market opportunities. The expansion of CAR T-cell therapies into solid tumors presents another growth avenue, as does the continued development of next-generation therapies that are more cost-effective and produce fewer side effects.
Recent Developments & Challenges
- Autolus Therapeutics Secures Funding Autolus Therapeutics has recently secured significant funding for the development of its next-generation CAR T-cell therapies. The company is focused on advancing its pipeline and expanding its offerings to include treatments for additional types of cancer.
- Juno Therapeutics' Approval for New Treatment Juno Therapeutics, a subsidiary of Bristol-Myers Squibb, has received FDA approval for a new CAR T-cell therapy for multiple myeloma. This approval is a significant milestone for the company and the CAR T-cell therapy market, as it opens up new treatment options for patients.
- Regulatory Changes in Asia In Asia, regulatory bodies are starting to approve CAR T-cell therapies, particularly in China and Japan. These approvals are expected to drive market growth, as access to cutting-edge therapies becomes more widespread.
- Challenges in Manufacturing Despite the promising future of CAR T-cell therapy, challenges remain in manufacturing and distribution. The process of collecting, modifying, and reintroducing T-cells can be complex and time-consuming, which limits scalability and increases treatment costs.
Key Players in the Market
- Autolus Therapeutics Autolus Therapeutics is a leading player in the CAR T-cell therapy market, with a focus on developing next-generation therapies. The company's proprietary technology platform allows it to engineer T-cells with superior cancer-fighting capabilities, targeting hematologic cancers and solid tumors. Autolus is committed to advancing its pipeline through ongoing clinical trials.
- CARsgen Therapeutics Co. Ltd. CARsgen Therapeutics is a Chinese biotech company focused on the development of innovative CAR T-cell therapies. The company is dedicated to advancing treatments for a range of cancers, including breast cancer, lung cancer, and hematologic cancers. Its novel approach to CAR T-cell therapies has garnered attention globally.
- Juno Therapeutics, Inc. Juno Therapeutics, a subsidiary of Bristol-Myers Squibb, is known for its CAR T-cell therapies targeting blood cancers. The company's lead products, including Kymriah, have revolutionized the treatment of certain cancers. Juno is continuously working to expand its portfolio and improve the effectiveness of its therapies.
- Gilead Sciences, Inc. Gilead Sciences is a major player in the CAR T-cell therapy market through its acquisition of Kite Pharma. Gilead’s Kymriah and Yescarta have become industry-leading products, providing effective treatment options for patients with hematologic cancers. The company is investing heavily in expanding its CAR T-cell therapy portfolio to include treatments for solid tumors.
Other key companies in the market include Bluebird bio, Inc., Sorrento Therapeutics, Inc., Novartis AG, and Celgene Corporation.
FAQs
Q1: What is CAR T-cell therapy? A: CAR T-cell therapy is a form of immunotherapy that modifies a patient’s T-cells to recognize and attack cancer cells. It has shown remarkable efficacy in treating blood cancers like leukemia and lymphoma.
Q2: How fast is the CAR T-cell therapy market growing? A: The CAR T-cell therapy market is expected to grow at a CAGR of 15.2% from 2025 to 2034, with the market size likely to reach USD 9.0 billion by 2034.
Q3: What are the challenges facing CAR T-cell therapy? A: Key challenges include high manufacturing costs, the complexity of the therapy process, and potential side effects such as cytokine release syndrome (CRS) and neurotoxicity.
Q4: Which regions are leading the CAR T-cell therapy market? A: North America leads the CAR T-cell therapy market, followed by Europe. Asia-Pacific is expected to see significant growth due to increased cancer prevalence and improved healthcare access.
Read Our Blog
Top 7 Companies in the Global Portable Medical and Healthcare Devices Market in 2025 - https://bitl.to/4B71
Top 7 Pediatric Medical Device Companies & Manufacturers Worldwide | 2025 - https://bitl.to/4B7w